Stem cell therapy has shown significant potential for treating joint problems, particularly in conditions like osteoarthritis, cartilage damage, and other musculoskeletal issues. Here's how stem cell therapy can help joints:
1. Regeneration of Cartilage
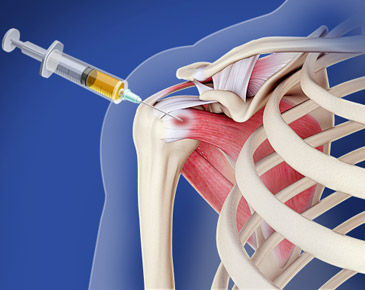
Cartilage Repair: Stem cells, particularly mesenchymal stem cells (MSCs) derived from bone marrow or adipose tissue, have the ability to differentiate into cartilage-producing cells (chondrocytes). When injected into damaged joints, they can stimulate the regeneration of cartilage, helping to restore joint function.
2. Reduction of Inflammation
Anti-inflammatory Effects: Stem cells release various growth factors and cytokines that help reduce inflammation in the joints. This can be especially beneficial for conditions like osteoarthritis, where chronic inflammation contributes to pain and damage.
3. Pain Relief
Pain Modulation: Stem cells can help modulate the pain signals in the joint area. This can result in reduced pain and improved mobility, as the cells promote the healing of damaged tissues and reduce inflammation, both of which are pain contributors.
4. Tissue Regeneration
Repair of Ligaments, Tendons, and Muscles: In addition to cartilage, stem cells can help repair other structures around the joint, such as tendons and ligaments. These tissues often suffer damage due to injury or chronic stress, and stem cells can assist in regenerating them, restoring joint stability and function.
5. Joint Function and Mobility
Restoration of Function: By promoting the regeneration of damaged tissues and reducing inflammation, stem cell therapy can improve overall joint mobility and function. Patients may experience increased range of motion, reduced stiffness, and improved strength.
6. Personalized Treatment
Customization: Stem cell therapy allows for a more tailored approach to treating joint issues. The treatment can be personalized based on the patient’s age, the extent of joint damage, and the type of tissue that needs repair.
7. Slowing Disease Progression
Long-Term Benefits: Stem cell therapy may slow the progression of degenerative joint diseases, such as osteoarthritis, by preventing further damage to cartilage and tissues. This can help maintain the health of the joint over time and delay the need for more invasive treatments like joint replacement.
Current Research and Considerations:
Although stem cell therapy for joint health is promising, it’s still being studied and is not universally approved or standardized for all patients or conditions.
Success rates vary, and not all patients respond the same way to treatment. The source of stem cells, the method of administration, and the specific joint being treated all play a role in the outcome.
.jpg)
Stem cell therapy holds great promise for cardiovascular diseases, offering the potential to regenerate damaged heart tissue, improve blood flow, reduce inflammation, and restore heart function. It represents an exciting avenue for treating chronic heart conditions, such as heart failure and coronary artery disease, that have limited treatment options. However, more research and clinical trials are necessary to fully understand the long-term effects and risks associated with these therapies. As the science advances, stem cells could play an increasingly important role in treating heart disease, improving patient outcomes, and reducing the need for heart transplants or other invasive interventions.
Stem cell therapy holds great potential for muscle regeneration, especially for conditions where muscle tissue is damaged, degenerating, or diseased. Muscle regeneration is a complex process that involves the repair of muscle fibers and the restoration of function. Stem cells can aid in this process by differentiating into muscle cells, promoting tissue repair, and potentially even reversing muscle damage caused by various conditions, such as injury, aging, or diseases like Duchenne Muscular Dystrophy (DMD). Here’s how stem cell therapy can contribute to muscle regeneration:
1. Differentiation into Muscle Cells
One of the most powerful aspects of stem cells is their ability to differentiate into specialized cells. In the case of muscle regeneration, muscle stem cells or pluripotent stem cells can be directed to become functional muscle cells.
Satellite Cells: Satellite cells are a type of muscle stem cell naturally present in muscle tissue. They are responsible for muscle repair and growth. When muscle fibers are damaged, satellite cells become activated and divide to form new muscle fibers. However, in chronic conditions or severe muscle damage (such as in muscular dystrophy), these cells may become exhausted or ineffective. By isolating and expanding these satellite cells, or introducing external stem cells to the muscle, regenerative therapies can boost the repair process.
Induced Pluripotent Stem Cells (iPSCs): iPSCs are created by reprogramming a patient's own somatic cells (e.g., skin or blood cells) into pluripotent stem cells. These cells have the ability to differentiate into any cell type, including muscle cells. iPSCs can be cultured and directed to differentiate into myocytes (muscle cells), which can then be transplanted into the damaged tissue to help regenerate muscle fibers.
2. Promoting Muscle Healing
Beyond differentiating into muscle cells, stem cells can release various growth factors and cytokines that promote muscle healing and repair. These molecules can stimulate the body’s own repair mechanisms, recruit additional stem cells to the site of injury, and reduce inflammation.
Growth Factors: Stem cells secrete a variety of growth factors that are crucial for muscle tissue regeneration. For example, factors like insulin-like growth factor (IGF-1), vascular endothelial growth factor (VEGF), and fibroblast growth factors (FGFs) help stimulate muscle cell proliferation, vascularization (new blood vessel formation), and tissue repair. These growth factors can accelerate the healing process and improve the integration of new muscle cells into the damaged tissue.
Anti-Inflammatory Effects: Muscle injury and disease often involve inflammation, which can further damage muscle tissue. Stem cells, particularly mesenchymal stem cells (MSCs), have shown the ability to modulate the immune response and reduce inflammation. By doing so, they can create a more favorable environment for muscle repair and regeneration.
3. Replacement of Damaged Muscle Tissue
In conditions like muscular dystrophies (e.g., Duchenne Muscular Dystrophy or Becker muscular dystrophy), muscle fibers are progressively replaced by scar tissue and fat. Stem cells can be used to replace the damaged muscle fibers and restore muscle function.
Transplantation of Stem Cells: Stem cells derived from the patient or a donor can be transplanted into the damaged muscle tissue. Once in place, these cells can differentiate into muscle cells, repair the muscle structure, and replace the degenerated muscle fibers. This helps restore muscle strength and function.
Mesenchymal Stem Cells (MSCs): MSCs can be injected into the muscle to aid in muscle regeneration. MSCs not only differentiate into muscle cells but also promote muscle healing by secreting factors that support tissue repair and reduce fibrosis (scar tissue formation).
4. Reversing Muscle Fibrosis (Scar Tissue)
Fibrosis, or the excessive formation of scar tissue, is a common feature of chronic muscle damage and diseases like DMD. Excessive fibrosis impairs muscle function and regeneration. Stem cells can help reduce fibrosis and improve the effectiveness of muscle regeneration.
MSC Therapy for Fibrosis: Mesenchymal stem cells (MSCs) have been shown to have antifibrotic effects, meaning they can inhibit the formation of scar tissue in damaged muscle areas. This is important because fibrosis often prevents the proper integration of new muscle fibers. By reducing fibrosis, stem cells can create a better environment for muscle regeneration.
Reduction of Inflammatory Response: MSCs can also modulate the immune system, reducing chronic inflammation, which is a contributing factor to fibrosis in muscles. This can help maintain the regenerative capacity of muscle tissue and promote healthier, less scarred muscle fibers.
5. Promoting Vascularization (Blood Flow)
Muscle regeneration requires an adequate blood supply to nourish the regenerating tissue. Stem cells can help promote the growth of new blood vessels, which is essential for muscle repair.
Vascular Endothelial Growth Factor (VEGF): Many stem cells, including MSCs, secrete VEGF, a protein that encourages the formation of new blood vessels (angiogenesis). This is important because new blood vessels supply oxygen and nutrients to the regenerating muscle tissue, enhancing its ability to heal and grow.
Enhanced Tissue Integration: By promoting vascularization, stem cells help ensure that the regenerated muscle tissue remains viable and fully integrated into the surrounding muscle, leading to better functional recovery.
6. Combining Stem Cells with Gene Therapy
Stem cells can be combined with gene therapy to treat underlying genetic causes of muscle degeneration. In diseases like Duchenne Muscular Dystrophy, the muscle degeneration is due to a genetic defect in the dystrophin gene. Stem cells can be genetically engineered to carry a corrected version of the gene and then transplanted into the patient’s muscles to restore the production of dystrophin, a key protein that maintains muscle integrity.
Gene Editing in Stem Cells: Stem cells can be genetically modified using tools like CRISPR-Cas9 to correct genetic mutations in muscle cells. Once these edited stem cells are transplanted into the muscle, they can regenerate functional muscle fibers and restore proper muscle function. This is particularly relevant in diseases like DMD, where correcting the underlying genetic defect is crucial to halting the progression of muscle damage.
7. Aging and Muscle Degeneration
As we age, our muscles lose mass and strength due to the depletion of satellite cells, the stem cells responsible for muscle repair. Stem cell therapy can help counteract this age-related muscle degeneration by replenishing the stem cell population in muscles and promoting regeneration.
Cell Therapy for Age-Related Muscle Weakness: Stem cells, particularly MSCs or iPSCs, can be used to stimulate muscle regeneration in elderly patients, restoring strength and function to muscles that have weakened over time. By rejuvenating the muscle stem cell pool, stem cell therapy can delay or even reverse the effects of age-related muscle loss.
8. Clinical Applications and Trials
Stem cell-based therapies for muscle regeneration are still in the experimental and clinical trial phases, but early results have been promising in conditions like muscular dystrophies, muscle injuries, and age-related muscle loss.
Clinical Trials: Several clinical trials are ongoing to assess the safety and effectiveness of stem cell therapies for muscle regeneration. Trials involving MSC injections or satellite cell transplantation have shown some success in improving muscle strength and function in both animal models and human patients.
Challenges and Limitations
While stem cell therapy for muscle regeneration shows significant promise, several challenges remain:
Engraftment and Integration: One of the key challenges is ensuring that the transplanted stem cells integrate properly into the muscle tissue and contribute to functional muscle repair.
Long-Term Effects: The long-term safety and effectiveness of stem cell therapies need to be thoroughly evaluated, as there is a risk of unwanted side effects, such as tumor formation or immune rejection.
Scalability and Cost: Producing stem cells in sufficient quantities and at an affordable cost for widespread clinical use remains a challenge.






